Jatia Mills: Aiming to improve the healing of traumatic brain injury

The following story was written in December 2021 by Joshua Vinson in ENGL 4824: Science Writing as part of a collaboration between the English department and the Center for Communicating Science.
You’re at Lane Stadium, cheering on the Virginia Tech Hokies. Just as the quarterback makes a pass to the wide receiver, he gets tackled from behind and his head slams against the ground. Dazed and confused, the quarterback lies on the field, groaning in pain. Trainers and medical personnel run to the player to assess his injuries. The quarterback is rushed to the hospital, having just experienced a moderate brain injury.
This is the reality for the more than 223,000 people who are hospitalized for traumatic brain injury (TBI) every year.[1] Many of these patients develop what is called a “secondary injury” following the initial impact, and Jatia Mills is working to improve the chances of a full recovery.
Mills, a second-year Ph.D. student at Virginia Tech, is researching the immunologic response following a moderate traumatic brain injury. She explained that there is an immediate need for understanding how the brain heals after moderate traumatic brain injury: “We want to figure out how we can step in or when [ . . . ] we can better treat traumatic brain injury so that we don't have people, especially [ . . . ] football players or sports players who are getting these head injuries and then later on, can't remember who they are.”
Born and raised in Prince George’s County, Maryland, Mills completed her undergraduate studies in biology at Morgan State University. During her undergraduate matriculation, she was chosen as a summer intern for the National Institute on Drug Abuse (NIDA) to study the effects of ketamine on major depression disorder. Following her studies, Mills did a 1-year post-baccalaureate research program at the University of Maryland’s School of Medicine. She investigated a new protein found to be involved in nurse shark immunology.
Mills’ research on nurse sharks piqued her interest in translational medicine, the practice of conducting research on lab animals and applying the results in clinical human and animal patients. Her background in both neuroscience and translational immunology led Mills to pursue her Ph.D. in Biomedical and Veterinary Sciences at the Virginia-Maryland College of Veterinary Medicine.
The Biomedical and Veterinary Sciences program allows her to combine both her neuroscience and immunology knowledge. Mills emphasized that her motivation for her Ph.D. studies stems from the potential for her research to help both humans and animals.
“I want for my work to be translational to both humans and animals,” she said, “and I want to be able to help both. From my perspective, you don't want to [conduct research on] animals just for humans. Personally, it doesn't feel good for me. If my work can help both, then that feels better for me.”
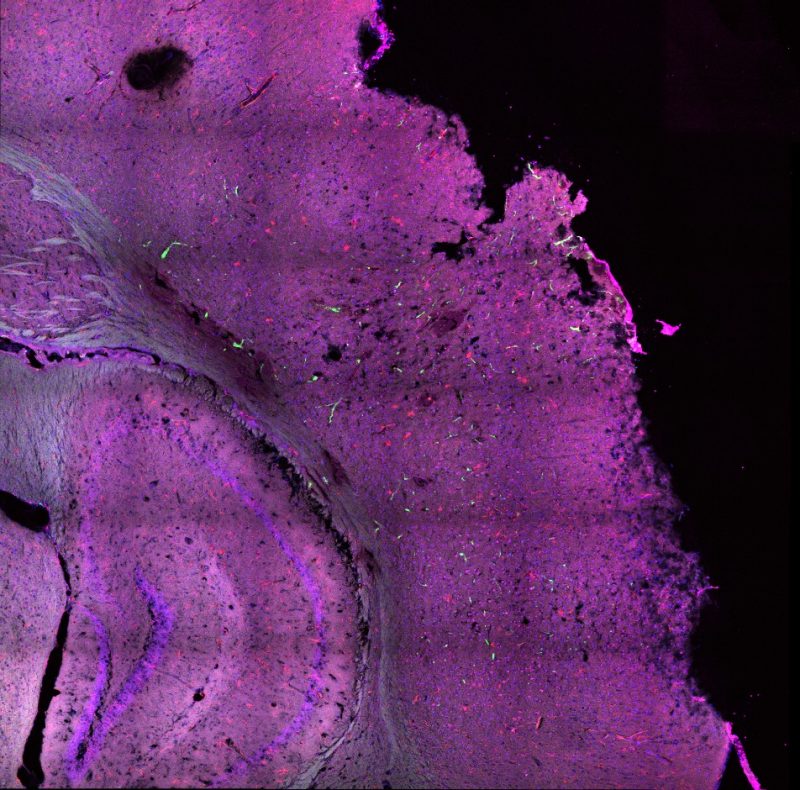
Mills works in Dr. Michelle Theus’ lab, conducting research on the immune response following traumatic brain injury in mice. After performing a cortical controlled injury on the mice, she collects the injured brain at different time points and analyzes the cellular interactions that follow the injury. By learning how these cellular interactions affect the brain’s healing, Mills will give doctors and veterinarians a sturdier foundation for how best to mediate traumatic brain injury before the development of a secondary injury.
Much about the brain is unknown, including how the brain fixes trauma in the central nervous system, known as neurological trauma. When the brain experiences moderate traumatic brain injury, the brain goes into an “urgency mode,” where immune cells within the central nervous system begin assessing damages. Immune cells from the peripheral (outer) nervous system are called to the scene to help the resident immune cells clean up the dead tissue and other harmful debris.
Among the cells arriving at the site of injury are microglia cells and peripheral immune cells. Microglia cells are resident to the central nervous system and are within the brain beginning with embryonic development. Peripheral immune cells are able to cross the blood-brain barrier once an injury occurs. When these two kinds of cells interact after a traumatic brain injury, the brain releases chemicals that increase inflammation, which can cause a secondary injury. Why does the immune system, despite its best effort to heal the brain’s injury, create more damage?
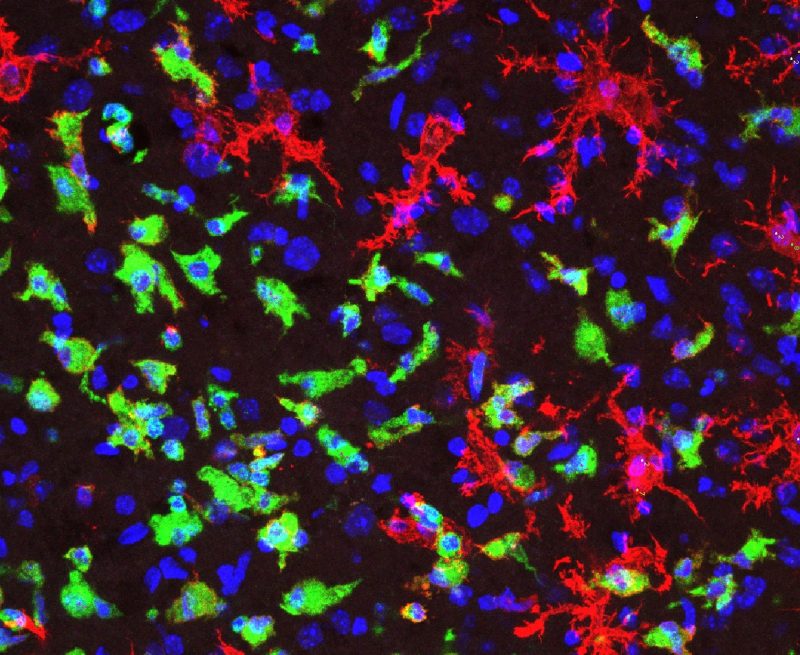
Mills elaborates on this issue, revealing why the brain experiences another injury: “It’s one thing [to] get a cut on your hand: it’ll get a scab, it’ll probably scar—normal healing procedure. But [the brain] is a lot more sensitive. What would be a normal healing [on our skin], in the brain is a lot more complicated and [leads] to more problems.” If Mills can identify how this interaction between microglia and peripheral immune cells affects the brain’s healing of traumatic brain injury, she will assist in developing treatments for more promising recoveries.
During these cellular interactions following traumatic brain injury, the microglia cells transform when they meet the harmful debris at the site of injury. Before this encounter, microglia cells are in a ramified (or branchlike) shape. When they detect the harmful debris, such as dead tissue, the microglia cells engulf (or “eat”) the dead tissue and change shape, morphing into an amoeboid (or orb-like) form.
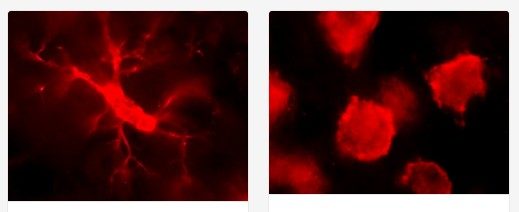
So far, Mills has found that at the three-day mark post-traumatic brain injury, there is an increase in the number of amoeboid-shaped microglia. She plans to investigate various temporospatial dynamics of both the resident and peripheral immune cells, including shape, cell division, and more to better understand the effects of these interactions.
While Mills’ team has not yet discovered how the interaction between microglia and peripheral immune cells influences the brain’s healing, they have made substantial progress in their research on the cellular response following moderate traumatic brain injury. Perhaps in the next couple of years, Mills’ team will make a breakthrough in their research. Then, the next time a Virginia Tech Hokies player experiences a traumatic brain injury at Lane Stadium, you can be assured that he will make a better recovery than before.
[1]CDC. (2018). TBI Data. In Traumatic Brain Injury and Concussion. Retrieved from Center for Disease Control and Prevention website: https://www.cdc.gov/TraumaticBrainInjury/data/index.html


