Blake Caldwell: Combating post-infection illnesses by targeting immune memory

The following story was written in December 2023 by Genevieve Donahue in ENGL 4824: Science Writing as part of a collaboration between the English department and the Center for Communicating Science.
After surviving a severe infection, the last thing on your mind is getting sick again. Sadly, for individuals who have battled certain life-threatening infections, post-infection illness is common. These additional infections can become a concern not long after a patient starts recovering from the original illness.
Combating and eliminating these post-infection conditions are the main goals of Blake Caldwell’s research.
“Looking at and understanding all of the building blocks in every single one of us… that’s what inspires me every day,” says Caldwell, a postdoctoral researcher in Liwu Li’s Laboratory of Innate Immunity & Inflammation Biology in Virginia Tech's Department of Biological Sciences. With a Bachelor of Science in biochemistry from Sewanee and a Ph.D. in cell and molecular biology from the University of Pennsylvania, Caldwell’s passion for science is fueled by understanding how organisms work and finding solutions to problems facing humanity.
His current research focuses on the long-term impacts on the immune system following sepsis or COVID-19 infection.
Sepsis is a condition caused by untreated infections that creates an intense response from the immune system. Without treatment, sepsis will lead to organ failure and death. According to the World Health Organization (2023), nearly 20 percent of all global deaths in 2017 were related to sepsis.
Treatment for sepsis has improved over the years, but complications remain.
“A lot of these recovered patients are left immunocompromised,” says Caldwell, “and so within a year's time, roughly 50 percent of these individuals will die due to secondary infection.”
Even after recovering from sepsis, some long-term effects may be present. One of these effects of sepsis is known as immunoparalysis, in which the immune system is stuck in an anti-inflammatory state, making the body more vulnerable (Schrijver et al, 2023).
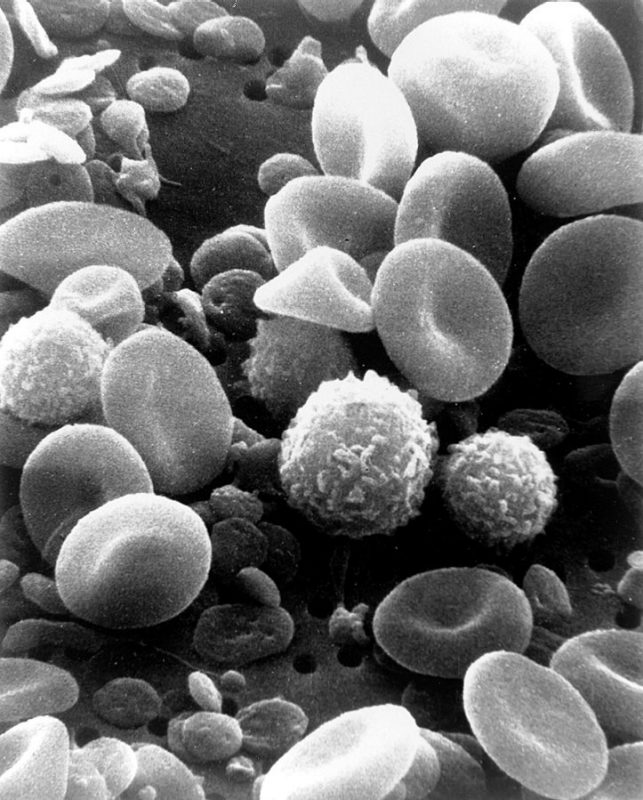
Caldwell’s research is focused on finding out what exactly causes this immune dysregulation and how it can be reversed. Monocytes, a type of white blood cell in the immune system, have an immune memory that can be altered by prolonged infection causing immune dysregulation, an effect known as monocyte exhaustion (Pradhan et al., 2023). Monocyte exhaustion is seen in septic infections as well as in severe COVID-19 infections, Caldwell explains.
Exhausted monocytes have high expression of pro-inflammatory genes, Caldwell says, which are important for telling the immune system to increase activity to eliminate the infection.
“But after the immune system resolves the infection, you really need the opposite, you need it to go back down,” says Caldwell. “But when monocytes are exhausted, they fail to bring those pro-inflammatory genes back down.” When this inflammatory state is retained for extended periods of time, it makes the body more vulnerable to further infections, he explains.
Eventually exhausted monocytes reach a state of paradoxical pro-inflammatory and anti-immune activity. During this state, the cells will have pro-immune functions such as inducing inflammation, expression of inflammatory cytokines, and production of heat, along with an anti-immune behavior in which the cells repress the activity of other immune cells.
“It's like spinning your wheels, basically; these cells are really bad at their job,” says Caldwell in describing this paradoxical state.
Exhausted monocytes may also de-differentiate into immature states and lose some of their normal abilities, such as removing bacteria or being able to differentiate into other types of immune cells.
“They de-differentiate and proliferate, which makes a lot of sense because when you're septic, you're having a dramatic amount of immune cell loss,” explains Caldwell. “It's a means of generating more immune cells to mount this immune response, but it just sort of goes too far.”
Caldwell and his team understand the phenomenon of monocyte exhaustion. The next step is to find out what exactly causes it. Fortunately, Caldwell’s graduate research, on a biochemical reaction called DNA methylation, provided him with valuable training to approach the problem. Using mouse sepsis models, the researchers have looked at the role of reversible and environmentally induced (epigenetic) changes to DNA in regulating the immune system, specifically innate immune memory.
“These are patients who have long-lasting immunodeficiency for sometimes years after the fact,” says Caldwell, “so there has to be some sort of a memory component there. Maybe this epigenetic memory might account for the failure of these monocytes.”
Caldwell’s research shows that monocyte exhaustion causes various changes in DNA methylation, especially increased methylation known as hypermethylation.
“It wasn't known to what degree DNA methylation contributed to any form of innate immune memory,” Cadwell explains, “so this evidence suggests that there's this major component of epigenetics that's been ignored in our field that people need to be paying more attention to.”
With data showing a variety of epigenetic changes caused by monocyte exhaustion as well as evidence of various therapeutic agents that could reverse these effects, Caldwell’s research provides initial steps for treating the long-term effects of sepsis and severe COVID-19 infection. Though he currently uses mice for his research, Caldwell’s work can lead to further research into treatments for human sepsis patients and survivors that would increase the probability of restoring proper immune function and increase their odds of survival.
One of Caldwell’s key motivators is “contributing positively to society to help these individuals live their best possible life.” With the increased understanding of the role of exhausted monocytes in reinfection, he is well on his way to that goal.
References
Breiling, A., & Lyko, F. (2015). Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics & Chromatin, 8(24). https://doi.org/10.1186/s13072-015-0016-6
Caldwell, B. A., Wu, Y., Wang, J., & Li, L. (2023). Altered DNA Methylation Underlies Monocyte Dysregulation and Innate Exhaustion Memory in Sepsis. https://doi.org/10.1101/2023.08.30.555580
Chen, J., Gao, L., Wu, X., Fan, Y., Liu, M., Peng, L., Song, J., Li, B., Liu, A., & Bao, F. (2023). BCG-induced trained immunity: History, mechanisms and potential applications. Journal of Translational Medicine, 21(1). https://doi.org/10.1186/s12967-023-03944-8
Moore, L. D., Le, T., & Fan, G. (2012). DNA methylation and its basic function. Neuropsychopharmacology, 38(1), 23–38. https://doi.org/10.1038/npp.2012.112
Pradhan, K. Yi, Z., Geng, S., & Li, L. (2021). Development of Exhausted Memory Monocytes and Underlying Mechanisms. Frontiers in Immunology, 12, 778830. https://doi.org/10.3389/fimmu.2021.778830
Schrijver, D.P., Röring, R.J., Deckers, J. et al. (2023). Resolving sepsis-induced immunoparalysis via trained immunity by targeting interleukin-4 to myeloid cells. Nature Biomedical Engineering, 7(9), 1097–1112. https://doi.org/10.1038/s41551-023-01050-0
World Health Organization. (2023, July 19). Sepsis. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/sepsis
Yoo, D. K., & Lee, S.-H. (2016). Effect of lipopolysaccharide (LPS) exposure on the reproductive organs of immature female rats. Development & Reproduction, 20(2), 113–121. https://doi.org/10.12717/dr.2016.20.2.113


